Why Is There No Change In The Temperature When The Process Of Melting Takes Place?
7.iii: Phase Changes
- Page ID
- 16107
- Decide the heat associated with a phase change.
Thing can exist in one of several different states, including a gas, liquid, or solid state. The amount of free energy in molecules of matter determines the state of matter.
- A gas is a state of matter in which atoms or molecules have plenty energy to movement freely. The molecules come into contact with ane some other simply when they randomly collide.
- A liquid is a land of matter in which atoms or molecules are constantly in contact but have enough energy to continue irresolute positions relative to ane another.
- A solid is a country of thing in which atoms or molecules do not have plenty free energy to motion. They are constantly in contact and in fixed positions relative to one another.
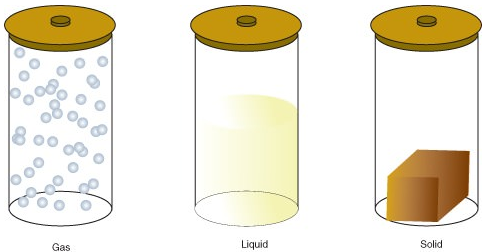
The post-obit are the changes of state:
| Solid → Liquid | Melting or fusion |
| Liquid → Gas | Vaporization |
| Liquid → Solid | Freezing |
| Gas → Liquid | Condensation |
| Solid → Gas | Sublimation |
- If estrus is added to a substance, such as in melting, vaporization, and sublimation, the procedure is endothermic. In this instance, heat is increasing the speed of the molecules causing them movement faster (examples: solid to liquid; liquid to gas; solid to gas).
- If heat is removed from a substance, such as in freezing and condensation, then the process is exothermic. In this instance, rut is decreasing the speed of the molecules causing them move slower (examples: liquid to solid; gas to liquid). These changes release heat to the surround.
- The amount of estrus needed to change a sample from solid to liquid would be the same to reverse from liquid to solid. The but divergence is the management of estrus transfer.
Label each of the following processes as endothermic or exothermic.
- h2o humid
- water ice forming on a pond
Solution
- endothermic - you must put a pan of water on the stove and give information technology heat in order to get h2o to boil. Considering y'all are calculation heat/free energy, the reaction is endothermic.
- exothermic - think of ice forming in your freezer instead. You put water into the freezer, which takes heat out of the h2o, to get information technology to freeze. Because heat is beingness pulled out of the water, it is exothermic. Heat is leaving.
Practise \(\PageIndex{1}\)
Label each of the following processes every bit endothermic or exothermic.
- water vapor condensing
- gold melting
- Answer
-
a. exothermic
b. endothermic
A phase change is a physical process in which a substance goes from one phase to another. Ordinarily the change occurs when adding or removing oestrus at a item temperature, known every bit the melting signal or the humid point of the substance. The melting point is the temperature at which the substance goes from a solid to a liquid (or from a liquid to a solid). The boiling point is the temperature at which a substance goes from a liquid to a gas (or from a gas to a liquid). The nature of the phase change depends on the direction of the heat transfer. Heat going into a substance changes information technology from a solid to a liquid or a liquid to a gas. Removing heat from a substance changes a gas to a liquid or a liquid to a solid.
Ii key points are worth emphasizing. Showtime, at a substance's melting betoken or boiling bespeak, two phases tin exist simultaneously. Take water (H2O) as an example. On the Celsius scale, H2O has a melting indicate of 0°C and a boiling signal of 100°C. At 0°C, both the solid and liquid phases of H2O can coexist. However, if heat is added, some of the solid HtwoO will melt and turn into liquid HtwoO. If heat is removed, the opposite happens: some of the liquid H2O turns into solid H2O. A similar process can occur at 100°C: calculation oestrus increases the corporeality of gaseous H2O, while removing rut increases the amount of liquid H2O (Effigy \(\PageIndex{1}\)).
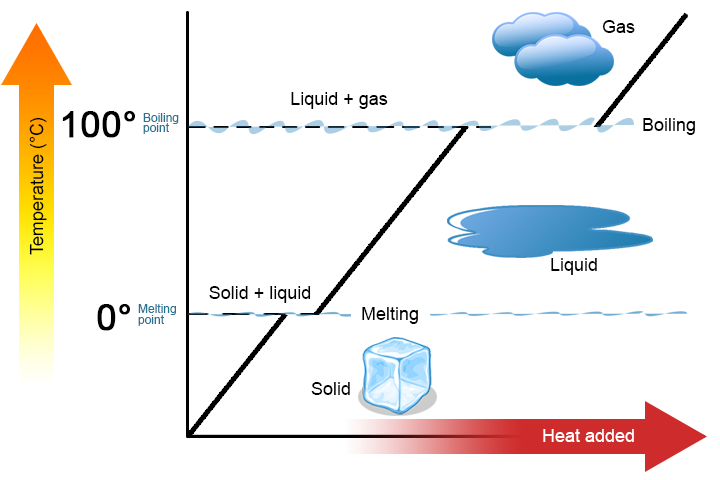
H2o is a good substance to use equally an example because many people are already familiar with information technology. Other substances accept melting points and boiling points as well.
Second, equally shown in Effigy \(\PageIndex{1}\), the temperature of a substance does not alter as the substance goes from one phase to another. In other words, phase changes are isothermal (isothermal means "constant temperature"). Over again, consider H2O every bit an instance. Solid h2o (ice) tin can exist at 0°C. If heat is added to ice at 0°C, some of the solid changes phase to make liquid, which is also at 0°C. Remember, the solid and liquid phases of HtwoO tin coexist at 0°C. Only after all of the solid has melted into liquid does the addition of heat change the temperature of the substance.
For each phase change of a substance, in that location is a feature quantity of heat needed to perform the stage change per gram (or per mole) of material. The heat of fusion (ΔH fus) is the corporeality of heat per gram (or per mole) required for a phase change that occurs at the melting betoken. The heat of vaporization (ΔH vap) is the amount of heat per gram (or per mole) required for a stage change that occurs at the boiling signal. If you lot know the total number of grams or moles of material, yous tin use the ΔH fus or the ΔH vap to determine the total oestrus being transferred for melting or solidification using these expressions:
\[\text{estrus} = n \times ΔH_{fus} \label{Eq1a}\]
wher east \(n\) is thursday eastward number of moles and \(ΔH_{fus}\) is expressed in energy/mole or
\[\text{estrus} = m \times ΔH_{fus} \label{Eq1b}\]
where \(m\) is the mass in grams and \(ΔH_{fus}\) is expressed in energy/gram.
For the humid or condensation, use these expressions:
\[\text{estrus} = north \times ΔH_{vap} \label{Eq2a}\]
wher e \(n\) is the number of moles) and \(ΔH_{vap}\) is expressed in energy/mole or
\[\text{heat} = m \times ΔH_{vap} \label{Eq2b}\]
wh ere \(m\) i due south the mass in grams and \(ΔH_{vap}\) is expressed in energy/gram.
Recall that a stage alter depends on the direction of the rut transfer. If estrus transfers in, solids become liquids, and liquids become solids at the melting and humid points, respectively. If rut transfers out, liquids solidify, and gases condense into liquids. At these points, there are no changes in temperature as reflected in the above equations.
How much estrus is necessary to cook 55.8 g of water ice (solid HtwoO) at 0°C? The rut of fusion of H2O is 79.9 cal/one thousand.
Solution
We can use the relationship between estrus and the heat of fusion (Equation \(\PageIndex{1}\)) to determine how many cal of heat are needed to cook this ice:
\[ \brainstorm{marshal*} \ce{rut} &= \ce{1000 \times ΔH_{fus}} \\[4pt] \mathrm{heat} &= \mathrm{(55.8\: \cancel{g})\left(\dfrac{79.ix\: cal}{\cancel{g}}\right)=four,460\: cal} \end{marshal*}\]
How much oestrus is necessary to vaporize 685 g of H2O at 100°C? The heat of vaporization of HtwoO is 540 cal/chiliad.
- Answer
-
\[ \begin{align*} \ce{heat} &= \ce{m \times ΔH_{vap}} \\[4pt] \mathrm{heat} &= \mathrm{(685\: \abolish{yard})\left(\dfrac{540\: cal}{\cancel{yard}}\correct)=370,000\: cal} \terminate{align*}\]
Table \(\PageIndex{i}\) lists the heats of fusion and vaporization for some common substances. Note the units on these quantities; when you use these values in problem solving, make sure that the other variables in your calculation are expressed in units consequent with the units in the specific heats or the heats of fusion and vaporization.
| Substance | ΔH fus (cal/g) | ΔH vap (cal/m) |
|---|---|---|
| aluminum (Al) | 94.0 | 2,602 |
| gold (Au) | xv.3 | 409 |
| iron (Fe) | 63.two | ane,504 |
| h2o (HtwoO) | 79.9 | 540 |
| sodium chloride (NaCl) | 123.v | 691 |
| ethanol (C2H5OH) | 45.ii | 200.3 |
| benzene (C6H6) | 30.4 | 94.1 |
There is also a phase alter where a solid goes directly to a gas:
\[\text{solid} \rightarrow \text{gas} \characterization{Eq3}\]
This stage change is called sublimation. Each substance has a characteristic rut of sublimation associated with this procedure. For instance, the heat of sublimation (ΔH sub) of H2O is 620 cal/yard.
We encounter sublimation in several ways. You lot may already be familiar with dry out ice, which is simply solid carbon dioxide (CO2). At −78.v°C (−109°F), solid carbon dioxide sublimes, changing directly from the solid phase to the gas phase:
\[\mathrm{CO_2(s) \xrightarrow{-78.5^\circ C} CO_2(g)} \label{Eq4}\]
Solid carbon dioxide is called dry ice because it does not pass through the liquid stage. Instead, it does straight to the gas phase. (Carbon dioxide can exist as liquid just only under high pressure.) Dry ice has many applied uses, including the long-term preservation of medical samples.
Fifty-fifty at temperatures beneath 0°C, solid H2O will slowly sublime. For example, a thin layer of snow or frost on the footing may slowly disappear as the solid HtwoO sublimes, fifty-fifty though the outside temperature may be below the freezing point of water. Similarly, ice cubes in a freezer may get smaller over time. Although frozen, the solid water slowly sublimes, redepositing on the colder cooling elements of the freezer, which necessitates periodic defrosting (frost-free freezers minimize this redeposition). Lowering the temperature in a freezer will reduce the need to defrost as often.
Under similar circumstances, water will also sublime from frozen foods (eastward.grand., meats or vegetables), giving them an unattractive, mottled appearance called freezer burn. It is not actually a "fire," and the food has not necessarily gone bad, although it looks unappetizing. Freezer fire tin can be minimized by lowering a freezer's temperature and by wrapping foods tightly so water does non have any space to sublime into.
Concept Review Exercises
- Explain what happens when heat flows into or out of a substance at its melting point or boiling point.
- How does the amount of heat required for a phase alter relate to the mass of the substance?
- What is the management of heat transfer in boiling water?
- What is the direction of oestrus transfer in freezing h2o?
- What is the management of rut transfer in sweating?
Answers
1. The free energy goes into changing the phase, not the temperature.
2. The amount of heat is a constant per gram of substance.
3. Boiling. Heat is beingness added to the water to get it from the liquid state to the gas country.
4. Freezing. Heat is exiting the system in guild to go from liquid to solid. Some other way to look at it is to consider the contrary process of melting. Energy is consumed (endothermic) to melt ice (solid to liquid) so the contrary process (liquid to solid) must be exothermic.
v. Sweating. Heat is consumed to evaporate the moisture on your skin which lowers your temperature.
Key Takeaway
- There is an energy change associated with any phase change.
Exercises
-
How much free energy is needed to melt 43.eight g of Au at its melting betoken of one,064°C?
-
How much energy is given off when 563.8 g of NaCl solidifies at its freezing point of 801°C?
-
What mass of ice tin be melted past 558 cal of energy?
-
How much ethanol (C2H5OH) in grams can freeze at its freezing indicate if ane,225 cal of rut are removed?
-
What is the rut of vaporization of a substance if 10,776 cal are required to vaporize 5.05 k? Limited your final answer in joules per gram.
-
If 1,650 cal of heat are required to vaporize a sample that has a heat of vaporization of 137 cal/grand, what is the mass of the sample?
-
What is the heat of fusion of water in calories per mole?
-
What is the heat of vaporization of benzene (Chalf-dozenHhalf dozen) in calories per mole?
-
What is the heat of vaporization of aureate in calories per mole?
-
What is the estrus of fusion of atomic number 26 in calories per mole?
Answers
-
670 cal
ii. 69,630 cal
-
viii,930 J/g
vi. 12.0 grand
-
1,440 cal/mol
8. 7,350 cal/mol
9. 80,600 cal/mol
10. three,530 cal/mol
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/The_Basics_of_GOB_Chemistry_%28Ball_et_al.%29/07:_Energy_and_Chemical_Processes/7.03:_Phase_Changes
Posted by: pylantantless.blogspot.com


0 Response to "Why Is There No Change In The Temperature When The Process Of Melting Takes Place?"
Post a Comment